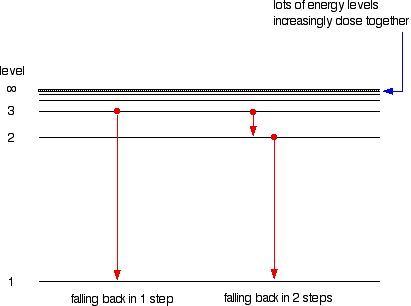
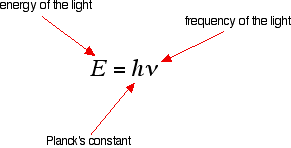
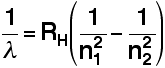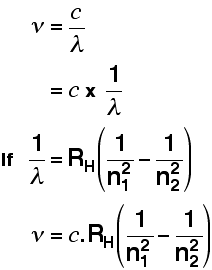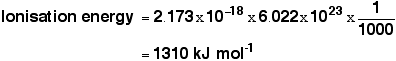Creating Films for Imax theatres
The IMAX theater experience is the leading cinema experience. The resolution, fidelity and stability of an original 15/70 motion picture image in an IMAX theater are a combination that nothing beats.

15/70 is the world's largest film format and was conceived as part of the development of the first IMAX projector (1970). For a filmmaker, large format film can be a creatively irresistible, but at the same time, highly challenging medium to work in.
15/70 is the most expensive medium in the world, so generally not the best format with which to learn how to make films. It can also be a difficult medium for experienced filmmakers who have learned their craft in another format and come to the larger format with expectations derived from their experience in the smaller, more versatile medium.
15 Perforation / 70mm
The 15/70 film frame is 10 times larger than a frame of 35mm used in regular cinemas and three times larger than standard 70mm film used in classic feature film releases such as “Lawrence of Arabia”. The 15/70 format is configured so that each frame is 15 perforations in width on 70mm film. Projected with an IMAX projector, the film runs horizontally through the gate (as compared with 35mm motion picture film and standard 70mm which run vertically through the projector).

The experience / Imax image capture
 The IMAX Camera
The IMAX Camera
The IMAX camera is designed to shoot the 15/70 film format. The IMAX camera uses 65mm negative film stock which is later contact printed to 70mm print stock (positive) for projection. The image area on the negative and on the print film stock is the same—the additional area on the 70mm format was originally designed to accommodate a soundtrack (optically or magnetically encoded).
IMAX cameras weigh between 42 and 100 pounds (19-45 Kg) approximately, depending on model and configuration. 3D film camera configurations weigh significantly more. A typical film load (one roll) is 1,000 feet (305 metres) in length and representsapproximately 3 minutes of raw footage.
 Alternate Formats
Alternate Formats
Other camera systems are sometimes used by filmmakers to capture scenes for use in films that will be exhibited on giant screens. These camera systems include 35mm and HD video. Typically scenes acquired on these lower resolution systems are digitally remastered to enhance their appearance for the big screen. IMAX Corporation offers a proprietary digital remastering service entitled DMR which is used principally to repurpose 35mm feature films for release in IMAX cinemas. Alternative formats cannot offer the full resolution and clarity possible with 15/70 film, but they do provide ways to create content that is not necessarily feasible with the larger and more expensive 15/70 format.
Imax Theatres
There are more than 400 large format theaters worldwide. Projectors manufactured by IMAX Corporation and using the 15/70 film format, represent the dominant technology in this marketplace.
What is IMAX? IMAX theaters combine advanced large format projection technology with giant screens up to 8 storeys high and powerful wrap-around 12,000-watt digital sound systems. The result is an unsurpassed sensory experience.


There are more than 280 IMAX theaters in 40 countries world wide. These theaters are located in commercial multiplex cinemas, in museums, science centers, aquaria, or theme parks, or are situated as stand-alone venues. Theater installations feature either IMAX (flat screen 2D) or IMAX 3D systems or the IMAX Dome system.
Click here to see more IMAX Theaters.
Imax Technology

IMAX, IMAX 3D and IMAX Dome projectors are the most advanced, precise and powerful film projectors ever built. IMAX Rolling Loop technology ensures far superior picture and focus steadiness to deliver the largest, sharpest and brightest images imaginable.
IMAX and IMAX 3D giant flat screens soar up to eight stories high and are designed to encompass the audience’s peripheral vision. These screens are painted silver to maximize the amount of light reflected back to the audience. Half the size of a football field and large enough to show a whale life-size, IMAX screens fully immerse audience members in the scene.
Semi-circular IMAX Dome screens produce huge images that soar and swoop above, beside and behind you to fully surround you in The IMAX Experience.
All these theaters project the 15/70 film format.
 Sound
Sound
IMAX uncompressed digital, wrap-around sound is simply unsurpassed in depth and clarity. A proprietary six-channel, Proportional Point SourceTM Loudspeaker system delivers exacting volume and quality at every seat throughout the theatre. Audience members hear every shade and subtlety, regardless of where they are sitting.
Other Formats and Manufacturers
Additional manufacturers of large format film projection systems include Simex-Iwerks and GOTO. While 15/70 is the principal format for projection in giant screen theaters, some theaters use 8/70 projection systems and a small number use 10/70 in planetarium environments. These film formats all use 70mm film but use different configurations of film frame.
Digital
Digital projection is finding its way into many conventional cinemas and it is only a matter of time before giant screen presentations will also be delivered this way. IMAX Corporation and other manufacturers are engaged in the development of digital projection technologies to deliver the substantially larger information load or ‘bandwidth’ required for giant screen presentations.
© 2010-2012 The Little Ganesha® All Rights Reserved.
 Subscribe in a reader
Subscribe in a reader




 15/70 is the world's largest film format and was conceived as part of the development of the first IMAX projector (1970). For a filmmaker, large format film can be a creatively irresistible, but at the same time, highly challenging medium to work in.
15/70 is the world's largest film format and was conceived as part of the development of the first IMAX projector (1970). For a filmmaker, large format film can be a creatively irresistible, but at the same time, highly challenging medium to work in. 
 The IMAX Camera
The IMAX Camera Alternate Formats
Alternate Formats

 IMAX, IMAX 3D and IMAX Dome projectors are the most advanced, precise and powerful film projectors ever built. IMAX Rolling Loop technology ensures far superior picture and focus steadiness to deliver the largest, sharpest and brightest images imaginable.
IMAX, IMAX 3D and IMAX Dome projectors are the most advanced, precise and powerful film projectors ever built. IMAX Rolling Loop technology ensures far superior picture and focus steadiness to deliver the largest, sharpest and brightest images imaginable. Sound
Sound