The Mariana Trench - Oceanography | |
| What is a Trench? At least 22 trenches have been identified although not all are classified as major. 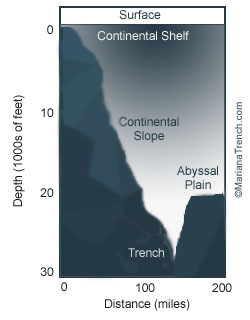 Of this number, 18 are in the Pacific Ocean, three in the Atlantic Ocean, and one in the Indian Ocean. Where, and What is the Mariana Trench? The Mariana Trench is located in the Pacific Ocean, just east of the 14 Mariana Islands (11"21' North latitude and 142" 12' East longitude ) near Japan. As you probably already know, it is the deepest part of the earth's oceans, and the deepest location of the earth itself. It was created by ocean-to-ocean subduction, a phenomena in which a plate topped by oceanic crust is subducted beneath another plate topped by oceanic crust. But just how deep is the Mariana Trench? First off, here are the average depths of the earth's oceans; the Arctic Ocean is 1,038 meters (3,407 feet) deep, the Indian Ocean is 3,872 meters (12,740 feet) deep, the Atlantic Ocean is 3,872 meters (12,254 feet) deep and the Pacific Ocean is 4,188 meters (13,740 feet) deep. The deepest point in each of the earth's oceans are as follows; the Arctic Ocean's Eurasian Basin at 5,450 meters (17,881 feet) deep, the Indian Ocean's Java Trench at 7,725 meters (25,344 feet) deep, the Atlantic Ocean's Puerto Rico Trench at 8,648 meters (28,374 feet) deep and the Pacific Ocean's Mariana Trench at 11,033 meters (36,201 feet) deep. The deepest point of the Mariana Trench is called The Challenger Deep , so named after the British exploration vessel HMS Challenger II, and it is located 210 miles south-west of Guam. This depth was reached in 1960 by the Trieste, a manned submersible owned by the U.S. Navy. In order to better illustrate the actual depth of the Mariana Trench, consider the following; if Mount Everest, which is the tallest point on earth at 8,850 meters (29,035 feet), were set in the Mariana Trench, there would still be 2,183 meters (7,166 feet) of water left above it. The Mariana Trench is often used as a North-South passage by submarines as it is part of a long system of trenches that circle the Pacific Ocean, connected with the Japan and Kuril Trenches. | |
Sunday, March 27, 2011
The Mariana Trench
Interesting Facts About Antarctica
1/ If Antarctica's ice sheets melted, the worlds oceans would rise by 60 to 65 metres (200 - 210ft) - everywhere. |
2/ Antarctica is pushed into the earth by the weight of its ice sheets. If they melted, it would "spring back" about 500m (1 625 ft). It would do this v...e...r...y s...l...o...w...l...y taking about 10000 years to do so. Scotland and Scandinavia are still rebounding today after the last ice age - at the rate of half a meter a century in the Northern Baltic - the fastest place. |
3/ Antarctica is the best place in the world to find meteorites. Dark meteorites show up against the white expanse of ice and snow and don't get covered by vegetation. In some places, the way the ice flows concentrates meteorites there. The ice makes them gather in one place. |
| 4/ The cold and dry conditions in the "Dry Valleys" region of Antarctica are so close to those on Mars that NASA did testing there for the Viking mission. It has not rained in the dry valleys for at least 2 million years. |
| 5/ One of the biggest icebergs ever (possibly the biggest iceberg ever) broke free from the Ross ice shelf in Antarctica in 2000.It was 295km (183 miles) long and 37km (23 miles) wide, with a surface area of 11,000 sq km (4,250 square miles) above water - and 10 times bigger below. It's similar in size to The Gambia, Qatar, The Bahamas, or Connecticut. |
| 6/ It has been estimated that during the feeding season in Antarctica, a full grown blue whale eats about 4 million krill per day (krill are small shrimp-like creatures), that's 3600 kg or 4 tons - every day for 6 months. Having laid down a layer of fat from this feeding activity in Antarctica, they then starve for several months.This daily intake would feed a human for about 4 years! If you could stomach it. Krill may be nutritious but they're not very nice as people food - which is lucky for the whales! |
| 7/ Since the Antarctic convergence arose about 20 million years ago, there has been very little exchange of fish or other marine life in either direction. This means that fish have lived in their side of the ocean and have not crossed over to their neighbours side. Antarctic fish have lived at between +2°C and -2°C for 5 million years (-2°C is the freezing point of sea water, below zero because of the salt). They are therefore the best cold adapted animals that there are on the planet - now or ever. |
| 8/ A domestic deep freeze runs at about -20°C. The mean summer temperature on the great East Antarctica icecap is -30°C and mean winter temperature around -60°C. That's a lot colder than your freezer!The lowest ever temperature recorded was at the Russian Vostok station. It was - 89.6°C |
| 9/ When the Antarctic sea-ice begins to expand at the beginning of winter, it advances by around 40,000 square miles (100,000 square kilometres) per day, and eventually doubles the size of Antarctica, adding up to an extra 20 million square kilometres of ice around the land mass.That's one and a half USA's, two Australia's or 50 UK's worth of ice area that forms, then breaks up and melts each year. |
| 10/ Snow falling at the South Pole takes about 100 000 years to "flow" to the coast of Antarctica before it drops off the end as part of an iceberg. |
| 11/ The Antarctic ice cap has 29 million cubic kilometres of ice. This is 90% of all the ice on the planet and between 60 and 70 % of all of the world's fresh water.Only about 0.4 percent of Antarctica is not covered by ice. |
| 12/ Antarctica has a peculiar group of fish called the ice fish. These have no red pigment - haemoglobin - in their blood to carry oxygen around. They get by perfectly well without it because the temperature is so low and oxygen dissolves better in cold temperatures. They just have a larger volume of clear blood instead and this gives them an unusually ghostly white colour, particularly their gills.Recent research on the ice fish ahs shown that their DNA has been damaged by high levels of ultra violet light coming from the ozone hole. They have less pigment to stop the UV getting through. Many other Antarctic sea creatures including fish have antifreeze in their blood so they don't accidentally get frozen solid! |
| 13/ The largest land animal in Antarctica is an insect, a wingless midge, Belgica antarctica, less than 1.3cm (0.5in) long. There are no flying insects (they'd get blown away), just shiny black springtails that hop like fleas and tend to live among penguin colonies. |
14/ Samples of ice known as ice cores are regularly drilled through the ice in Antarctica by scientists. They are removed as a long cylinder of ice that gives an indication of the past going back tens of thousands of years. The properties of the ice, of dust trapped in the ice, and even of air bubbles trapped in the ice give valuable information about the earth's climate at various times in the past. A glaciologist could easily give you a drink of water that was frozen during the life of Christ. |
| 15/ In 1981 a swarm of krill was tracked by US scientists that was estimated at being up to 10 million tonnes of krill! This is the equivalent of about 143 million people (at an average of 70kg each) or more than the entire populations of the UK and Germany combined ( and wandering around in a group!) |
| 16/ Antarctica is the only continent with no indigenous species of ants. |
What is a Share ?
In finance a share is a unit of account for various financial instruments including stocks, mutual funds, limited partnerships, and REIT's. In British English, the usage of the word share alone to refer solely to stocks is so common that it almost replaces the word stock itself.
In simple Words, a share or stock is a document issued by a company, which entitles its holder to be one of the owners of the company. A share is issued by a company or can be purchased from the stock market.
By owning a share you can earn a portion and selling shares you get capital gain. So, your return is the dividend plus the capital gain. However, you also run a risk of making a capital loss if you have sold the share at a price below your buying price.
A company's stock price reflects what investors think about the stock, not necessarily what the company is "worth." For example, companies that are growing quickly often trade at a higher price than the company might currently be "worth." Stock prices are also affected by all forms of company and market news. Publicly traded companies are required to report quarterly on their financial status and earnings. Market forces and general investor opinions can also affect share price.
Quick Facts on Stocks and Shares
- Owning a stock or a share means you are a partial owner of the company, and you get voting rights in certain company issues
- Over the long run, stocks have historically averaged about 10% annual returns However, stocks offer no
guarantee of any returns and can lose value, even in the long run - Investments in stocks can generate returns through dividends, even if the price
How does one trade in shares ?
Every transaction in the stock exchange is carried out through licensed members called brokers.
To trade in shares, you have to approach a broker However, since most stock exchange brokers deal in very high volumes, they generally do not entertain small investors. These brokers have a network of sub-brokers who provide them with orders.
The general investors should identify a sub-broker for regular trading in shares and palce his order for purchase and sale through the sub-broker. The sub/broker will transmit the order to his broker who will then execute it .
What are active Shares ?
Shares in which there are frequent and day-to-day dealings, as distinguished from partly active shares in which dealings are not so frequent. Most shares of leading companies would be active, particularly those which are sensitive to economic and political events and are, therefore, subject to sudden price movements. Some market analysts would define active shares as those which are bought and sold at least three times a week. Easy to buy or sell.
The Most Powerful Indians In INDIA
1. SH KAPADIA ( Age 63 )
WHY
He is number one in the list because as CHIEF JUSTICE OF INDIA. He represents the Supreme Court's power.At a time when so much of Politics is being determined by judicial pronouncements and even observations - related to headline - grabbing court cases, the number one judge and the court he runs have become the country's most influential arbiter. His and his court's decisions can determine how Govt. and Politics are affected by the 2G scandal, or whether the CVC, PJ Thomas, will go and take a big chunk of UPA credibility with him. The SC will also take significant decisions on the privacy vs public interest debate and perhaps on industrial projects vs environmental laws too. The apex court has never been as hugely crucial in determining policy and politics as in recent times. Therefore, a Chief Justice of INDIA has never been as popular as he is now.
click on read more for further info about top 10 most powerful Indians
POWER PUNCH
He took less than a minute to decide, after hearing lengthy arguments, that the Allahabad High Court's verdict on the Ayodhya Title suit must be pronounced; threats of violence could not be an excuse.
WHAT NEXT
Court business wise, his word on the CVC will be the first of many Judicial calls this year.
Administration wise, he is keen to provide decent infrastructure to the lowest courts.
BY THE WAY : He once refused an invitation to open a judicial conference abroad because the event fell on a working day.
2. SONIA GANDHI ( Age 64 )
WHY
She goes up one place in the list despite the Congress led Govt.'s many troubles because with the PM looking less than effective, the Party Leader's job has become even more crucial, not the least in terms of keeping critics of the PM at bay.She prevailed upon DMK to remove A Raja. The National Advisory council (NAC), under her leadership, remains an important pressure group.
POWER PUNCH
She took tough calls on sacking Congress notables embroiled in controversies, Shashi Tharoor and Ashok Chavan being the Prime examples, and challenged the BJP to take similar action against its leaders accused of corruption.
WHAT NEXT
Nothing less than the immediate future of the Congress. Elected, inevitably, as Congress President again, and the term extended from three to five years, she will lead the party beyond the 2014 elections.
BY THE WAY :: She often uses SMS to keep in touch with the senior party leaders and ministers.
3. MANMOHAN SINGH ( Age 78 )
WHY
The PM drops one palce after convincingly winning a second term because the Govt. has looked indecisive and riddled with internal fights. The economy has held up, but most of what he wanted to do continues to be in the pending tray. The NAC frequently questions the Govt and there appear to be question marks over his authority i9n the Cabinet. He's held his nerve, given the circumstances, but insiders feel the pressure might tell. Still, he remains the Congress's best bet as Govt.'s face to the country and the world.
POWER PUNCH
His foreign policy credentials keep growing-- in a span of three months last year, leaders of all the P-5 countries visited India. That's a first fao an Indian PM.
WHAT NEXT
Crisis management, definitely, one part of which will be an effective Cabiner reshuffle. Also, economic policy is in drift and needs the attention of the "original economic reformer".
BY THE WAY :: There's a special bed for him abroad Air India One but he never uses it.Insists on half-an-hour on the treadmill, no matter where he is.
4. SUSHMA SWARAJ (LEADER OF OPPOSITION, LOK SABHA)
5. RAHUL GANDHI (CONGRESS GENERAL SECRETARY )
6. NITISH KUMAR ( BIHAR CHIEF MINISTER)
7. MAMATA BANERJEE (RAILWAYS MINISTER)
8. P CHIDAMBARAM (UNION HOME MINISTER)
9. MUKESH AMBANI (CHAIRMAN & MANAGING DIRECTOR, RELIANCE INDUSTRIES)
10. PRANAB MUKHERJEE (UNION FINANCE MINISTER)
11. D SUBBARAO ( R.B.I GOVERNOR)
12. JAIRAM RAMESH (UNION ENVIRONMENT MINISTER)
13. ARUN JAITLEY (LEADER OF OPPOSITION, RAJYA SABHA)
14. AK ANTONY (DEFENCE MINISTER)
15.MS DHONI ( INDIA CRICKET CAPTAIN )
16.J JAYALALITHA ( AIDMK LEADER)
17. AHMED PATEL ( POLITICAL SECRETARY TO SONIA GANDHI )
18. NARENDRA MODI ( GUJARAT CM )
19. AZIM PREMJI ( WIPRO CHAIRMAN)
20. JAGANMOHAN REDDY ( POLITICIAN )
21. SACHIN TENDULKAR ( CRICKETER)
22.PRITHVIRAJ CHAVAN ( MAHARASHTRA CM)
23. KAPIL SIBAL ( UNION HRD AND TELECOM MINISTER)
24. RAMAN SINGH ( CHATTISGARH CM)
25. SYED ALI SHSH GILANI ( HURRIYAT LEADER )
26. RAHUL BHATIA ( CHAIRMAN, INDIGO AIRLINES)
27. NC SAXENA (MEMBER, NATIONAL ADVISORY COUNCIL)
28. K CHANDRASEKHAR RAO (TRS PRESIDENT)
29. DIGVIJAY SINGH (CONGRESS GENERAL SECRETARY )
30.NITIN GADKARI ( BJP PRESIDENT)
31. ANAND SHARMA (UNION COMMERCE & INDUSTRIES MINISTER )
32. AMAR PRATAP SINGH ( CBI DIRECTOR )
33. SHIVSHANKAR MENON ( NATIONAL SECURITY ADVISER)
34. SALMAN KURSHID ( UNION WATER RESOURCES AND MINORITY AFF. MINISTER)
35. SUNIL MITTAL ( CHAIRMAN & GRP. CEO, BHARTI ENTERPRISES)
36.MAYAWATI ( UP CM)
37. L K ADWANI (BJP PARLIAMENTARY PARTY CHAIRMAN )
38. KALANIDHI MARAN ( CHAIRMAN & MANAGING DIRECTOR, SUN NETWORK )
39. RATAN TATA (TATA GRP. OF CHAIRMAN )
40. GANAPATHI (CPI MAOIST GEN. SECRETARY )
41. DEEPAK PAREKH ( HDFC CHAIRMAN )
42. MOHAN BHAGWAT ( RSS SARSANGHCHALAK )
43. NANDAN NILEKANI ( CHAIRMAN,UNIQUE IDENTIFICATION AUTHORITY OF INDIA)
44. GOOLAM E VAHANVATI (ATTORNEY-GEN. OF INDIA)
45. SY QUARAISHI (CHIEF ELECTION COMMISSIONER )
46. KAUSHIK BASU (CHIEF ECONOMIC ADVISER)
47. CHANDA KOCHHAR ( MD & CEO, ICICI BANK )
48. FALI S ANRIMAN ( LEGAL EXPERT )
49. GM RAO (CHAQIRMAN GMR GROUP)
50. SHARAD PAWAR ( UNION AGRI. MINISTER )
51. ANIL AMBANI ( CHAIRMAN, ANIL DHIRUBHAI AMBANI GRP.)
52. VEERAPPA MOILY ( UNION LAW MINISTER )
53. NAVEEN PATNAIK (ORISSA CM )
54. KAMAL NATH ( UNION URBAN DEV. MINISTER )
55 .HR BHARADWAJ ( GOVERNOR OF KARNATAKA )
56. SAMIR JAIN (VICE-CHAIRMAN, BENNETT COLEMAN & CO.)
VINEET JAIN ( MD, BENNETT COLEMAN & CO.)
57. E SREEDHARAN ( CHIEF, DELHI METRO)
58. ESL NARASIMHAN ( GOVERNOR OF A.P )
59.BRIJMOHAN LALL MUNJAL (HERO HONDA CHAIRMAN)
60. MK STALIN (DY. CM TAMIL NADU )
61. PRASHANT BHUSHAN ( SENIOR LAWYER)
62. SUSHIL KUMAR MODI ( DY. CM BIHAR)
63. AMIT MITRA ( FICCI SEC.-GEN.)
64. SUBIR GOKARN ( DY. GOV. OF RBI )
65.ANAND MAHINDRA (MAHINDRA&MAHINDRA VICE-CHAIRMAN & MNG. DIR. )
66. KV KAMATH (ICICI BANK CHAIRMAN)
67.RAJ THACKERAY ( MNS PRESIDENT)
68. AAMIR KHAN (ACTOR, PRODUCER)
69. GHULAM NABHI AZAD (HEALTH MINISTER)
70. GEN. VK SINGH (CHIEF OF ARMY STAFF)
71.MK NARAYAN (GOV. OF BENGAL )
72. VINOD RAI ( COMPTROLLER & AUDITOR GEN. OF INDIA)
73. N RAM ( EDITOR-IN-CHIEF, THE HINDU)
74. KUMAR MANGALAM BIRLA (ADITYA BIRLA GRP.)
75.LAKSHMI NIWAS MITTAL ( CHAIRMAN & CEO ,ARCELOR MITTAL )
76. SHEILA DIKSHIT ( CM DELHI )
77.SHOBHANA BHARTIA ( CHAIR-PRSN. & EDIT. DIR. OF HT MEDIA )
78. SALMAN KHAN (ACTOR)
79.AVEEK SARKAR (DIR. & CHIEF EDITOR )
80.SHIVRAJ SINGH CHOUHAN (MP CM)
81. KARAN JOHAR ( DIRECTOR,PRODUCER)
82. SANJAY GUPTA (CEO JAGRAN PRAKASHAN)
MM GUPTA ( CHAIRMAN & MD, JAGRAN PRAKASHAN )
83. UDAY KOTAK ( VICE-CHAIRMAN & MD, KOTAK MAHINDRA GRP.)
84. VENU SRINIVASAN
MALLIKA SRINIVASAN ( TVS MOTOR CO. CHAIRMAN & MD, TAFE DIRECTOR )
85. UDAY SHANKAR ( CEO, STAR INDIA )
86. ARUN SHOURIE ( BJP LEADER)
87. OMAR ABDULLAH (JAMMU & KASHMIR CM)
88. SHASHANK MANOHAR ( BCCI PRESIDENT )
89. JAWAHAR SIRCAR ( SECRETARY, MIN. OF CULTURE)
90. ARNAB GOSWAMI ( TIMES NOW ,EDITOR-IN-CHIEF)
91. DEVI PRASAD SHETTY (CARDIAC SURGEON&CHAIRMAN, NARAYANA HRUDALAYA HOSPITALS)
92.RAGHAV BAHL (FOUNDER & MANAGING DIR. ,NETWORK 18 )
93.SRI SRI RAVI SHANKAR ( ART OF LIVING , FOUNDER)
94. UK SINHA ( CHAIRMAN & MNGGNG. DIR.,UTI-AMC)
95.KAREENA KAPOOR ( ACTRESS)
96. RAJIV BAJAJ ( MD, BAJAJ AUTO )
97. JAGGI VASUDEV ( GURU, ISHA FOUNDATION)
98. K CHANDRASEKHAR ( CABINET SECRETARY )
99. KATRINA KAIF ( ACTRESS)
100.BELLARY BROTHERS
G JANARDHAN REDDY, G KARUNAKARA REDDY, G SOMASHEKHAR REDDY
Thursday, March 24, 2011
Biography of most famous magician Harry Houdini
Early Life
Throughout his life, Harry Houdini claimed that he was born April 6, 1874 in Appleton, Wisconsin. In fact, he was born with the name Ehrich Weisz on March 24, 1874, in Budapest, Hungary. His father was Mayer Samuel Weisz, a religious teacher, whose first wife had died in childbirth. Ehrich was a child of his second wife, Cecilia Steiner. How many children the couple had is unclear, although six of their children survived to adulthood. Hoping for a better life for his family, Mayer emigrated to America and changed the spelling of his last name to Weiss. Through a friend, he gained a job serving as a rabbi to a small Jewish congregation in Appleton, with an annual salary of $750. His family is believed to have followed him to America in 1876, when Ehrich was a toddler. Stories of Ehrich performing magic and escape tricks while in Appleton have never been verified. His mother claimed that as a child he learned to open locked cabinets to get at pies and sweets she had baked, but the story may be more legend than fact.Mayer Weiss’s religious views were considered old-fashioned by the Appleton congregation and after a few years he was dismissed from his post. The family moved to Milwaukee when Ehrich was about eight, but times were difficult. From a young age, Ehrich sold newspapers and shined shoes to help support the family. When not working, Ehrich engaged in athletic activities and practiced acrobatic stunts. Ehrich claimed October 28, 1883 as the date of his first appearance before an audience. The nine year-old performed on a trapeze hung from a tree while wearing red socks made by his mother. He billed himself as “Ehrich, the Prince of the Air.”
At age 12, Ehrich ran away from home by hopping a freight car. The train took him to Kansas City, but where else he may have gone, and what he did during that time, is not known. A year later he re-joined his family, now living in New York City but still struggling to survive. Ehrich continued to work at a variety of jobs, including messenger, necktie cutter, and photographer’s assistant. At about this time, Ehrich and his younger brother Theo began to pursue an interest in magic. Ehrich’s idol was the great French magician Robert-Houdin. When Ehrich started performing magic before small groups, he added an “i” to the end of his hero’s name and called himself “Houdini.” The “Harry” is most likely an American version of his childhood nickname Ehrie.
Professional Career
Harry Houdini began his professional career at age 17 doing magic shows before civic groups, in music halls, at sideshows, and at New York’s Coney Island amusement park, where he sometimes performed 20 shows each day. For a time he worked with his brother Theo as The Houdini Brothers. This changed when Harry met Beatrice Raymond, a teenaged singer and dancer who was also attempting a career in show business. Harry and Bess married in 1894 and Bess joined the act as Harry’s new partner. (Theo started a solo career as a magician under the name Hardeen.) Harry and Bess remained devoted companions for the rest of his life. He depended on her to care for him and handle the necessities of life. Harry gave her the credit for his success, and developed the habit of writing her a love note every day. In 1895, the Houdinis joined the Welsh Brothers Circus for six months. Harry did magic, Bess sang and danced, and together they performed a trick called “Metamorphosis,” in which they switched places in a locked trunk. Not satisfied with the small scale of the act, Harry continued to work on new tricks and to develop his speaking voice and showmanship. He also became an expert at handcuffs. Arriving in a new town, Houdini would claim the ability to escape from any handcuffs provided by the local police. His easy escapes provided excellent publicity for his shows. Houdini offered $100 to anyone who provided handcuffs from which he could not escape, but he never had to pay. Through his increasingly complex escapes and his shrewd use of publicity, Houdini became a headliner on the vaudeville circuit, playing in cities across the country. Not satisfied with that low level of fame, however, Houdini decided to gamble by taking his act to Europe.
In 1900, Harry and Bess sailed to England with no bookings and only enough money to survive a week. Houdini was able to get an engagement at a London theater, but his breakthrough came when he successfully broke free after being wrapped around a pillar and handcuffed at Scotland Yard. The publicity from that escape caused the theater to extend Houdini’s booking. His fame quickly spread and he eventually played there for six months. Sold-out engagements quickly followed in Germany and then throughout Europe. Wherever he went, Houdini called upon local police to restrain him, but he continually confounded the authorities and escaped. To increase publicity, he also jumped into rivers while handcuffed and chained. Allowing the suspense to build, Houdini remained underwater long after many observers were certain he couldn’t survive, only to spring up, waving the chains over his head.
By the time Houdini returned to the United States in 1905, he was an international celebrity. Among the stunts performed to publicize his American appearances, Houdini escaped from the prison cell that held the assassin of President James Garfield, squirmed from a straitjacket while hanging upside down, and broke free from a packing crate that had been nailed shut and immersed underwater. This showmanship also extended to his act. As a regular feature of his performances, Houdini was shackled and lowered into an oversize milk can filled with water and then hidden by a curtain. Though he was usually able to escape in three minutes, Houdini frequently stayed behind the curtain for up to a half hour, making his re-appearance all the more dramatic. On one occasion in England, Houdini allowed the milk can to be filled with beer rather than water. As someone who never drank alcohol, Houdini was not used to the effects of the beer and had to be pulled to safety by his assistants. It was one of his rare failures.
Houdini the Man
Houdini was able to perform his difficult feats by remaining in excellent physical and mental condition. He pushed himself relentlessly. To develop his capacity for holding his breath, Houdini installed an oversize bathtub in his house so that he could practice regularly. Through extensive training, he was able use his left hand nearly as well as his right. While casually chatting with friends, he would perform card and coin tricks without looking at his hands, or tie and untie knots in pieces of rope with his feet. Determined to stay on top of the entertainment field, Houdini refined techniques he had already mastered and continually developed new and more daring escapes.As his reputation grew, Houdini assumed a leadership role among other magicians. He served as president of the Society of American Magicians and founded the Magician’s Club in London. Houdini was generous with other magicians, but jealous of anyone who attempted to duplicate his escapes. He wrote books and magazine articles that revealed some of magic’s simpler tricks, but carefully guarded his own secrets. Though known to be friendly and warm, Houdini had a large ego, could be touchy and petty at times, and frequently displayed a volatile tempter to his assistants.
In 1909, just six years after the Wright brothers proved that human flight was possible, Houdini became fascinated with airplanes. He bought his own plane, and learned to drive a car solely in order to get to the airport faster. In 1910, he became the first to successfully fly a plane in Australia. After that flight, however, his interest ended and he never piloted a plane or drove a car again. Houdini was also a great collector, with extensive collections of locks, magic memorabilia, autographs, historical items and, especially, books. Houdini collected so many books that he hired a full-time librarian to care for them, and traveled with hundreds at a time.
When America entered the First World War in 1917, Houdini tried to enlist in the army, but was rejected as being too old at age 43. Unable to fight, Houdini preformed free shows for service men, during which he would produce five dollar gold pieces from the air and toss them to the audience. He claimed to have distributed $7,000 in that manner. Houdini also organized shows in support of Liberty Bonds to help finance the war.
After the war, Houdini became an actor, appearing in a 13-part silent film serial called The Master of Mystery. The series was sufficiently successful that Houdini was hired to make two feature films. When those films performed poorly at the box office, Houdini blamed the movie company and opted to make his own movies. He formed a production company with his brother Theo, and controlled every aspect of his next two films, The Man from Beyond and Haldane of the Secret Service. Like his earlier movies, they featured daring stunts and escapes, but also like the earlier movies, they were not successful. Though some of the action sequences were thrilling, critics panned Houdini’s wooden acting and ineffective love scenes. He was so embarrassed at having to kiss another woman onscreen that he gave his wife five dollars every time he did so. Accepting defeat, Houdini gave up on the film business.
When not traveling, Harry and Bess lived in a large house they purchased in New York. The couple had no children, but Harry’s mother lived with them. Houdini was very close to his mother, and her death in 1913 was the greatest tragedy of his life. For weeks after her death, he made almost daily visits to the cemetery, sometimes lying on her grave to speak to her. “My mother was everything to me,” he said in a speech to the Magician’s Club. “It seemed the end of the world when she was taken from me…All desire for fame and fortune had gone from me. I was alone with my bitter agony…” Eventually, Houdini was able to return to work, but he continued to mourn his mother for the rest of his life.
Spiritualism
Partly as a result of his mother’s death, Houdini renewed an early interest in spiritualism, the so-called ability to communicate with the dead. Houdini wanted to believe that such communication was possible, but after many years performing magic, he was familiar with the methods employed by phony spiritualists to fool the public. Passing up better-paying opportunities, Houdini lectured on the subject of fraudulent spiritualists and unmasked many in the cities he visited. In his act, Houdini demonstrated many of the tricks used by spiritualists and wrote a best-selling book, A Magician Among the Spirits, which detailed their deceptions. Houdini had a standing offer of $10,000 to anyone who could produce a psychic effect that couldn’t be reproduced by natural means, but no one ever collected the money. Houdini so strongly opposed the phony spiritualists that he testified against them before a committee of Congress. “Please understand that, emphatically, I am not attacking a religion,” he said. “I respect every genuine believer in spiritualism or any other religion…But this thing they call spiritualism, wherein a medium intercommunicates with the dead, is a fraud from start to finish...In thirty-five years, I have never seen one genuine medium.”Because of his interest in spiritualism, Houdini developed a friendship with Sir Arthur Conan Doyle, author of the Sherlock Holmes stories, who was a firm believer in spiritualism. Conan Doyle was convinced that psychic powers enabled Houdini to perform his stunning escapes, and refused to accept Houdini’s denials and explanations. Eventually their disagreement over spiritualism and psychic ability led to an estrangement. The friendship ended as they attacked each other publicly.
The Last Days
In the fall of 1926, Houdini took a new show on the road. It was an elaborate, two and half hour performance, requiring Houdini to be on stage almost the entire time. The show featured magic, a section debunking spiritualism, and escapes from a coffin and a Chinese water torture, which had become one of Houdini’s most famous stunts. In the Chinese water torture escape, Houdini’s hands and feet were bound and he was lowered, upside down, into a glass tank filled with water, which was then securely closed. In mid-October, the tour took a bad turn in Providence, Rhode Island when Bess contracted a case of food poisoning. Despite the presence of a nurse, Houdini was deeply worried about his wife and stayed awake all night at her side. By the time they reached the next stop, Albany, New York, Houdini had gone three nights without sleep, his only rest coming from brief naps. Then, during the Albany show, the frame holding his leg in place for the Chinese water torture jerked, causing his ankle to break. Used to performing with smaller injuries, Houdini refused medical care and insisted on completing the show, but was awake all night from the pain. The tour nonetheless proceeded to the next stop in Montreal, Canada.Ignoring a doctor’s advice to stay off his foot, Houdini stuck to his schedule, including a lecture at McGill University. While there, Houdini met an art student who presented him with a sketch he had made of the great escape artist. Houdini invited the student to visit him backstage before the afternoon performance of his show. The next day, the student and two friends were chatting with Houdini in his dressing room when one of the students, an amateur boxer, asked if it was true that Houdini could withstand any blow to his body above the waist, excluding his face. Houdini admitted that it was true and, despite his weakened state due to his injury and lack of sleep, gave the student permission to test him. Houdini began to rise from the couch where he was seated, but before he had time to tighten his abdomen muscles, the student punched him three times in the stomach. Houdini fell back on the couch, his face white. Although in pain, Houdini performed his show that afternoon. The pain was worse in the evening, but Houdini refused to consult a doctor.
The next day, October 24, despite chills and sweating, Houdini performed two more shows before the company moved on to Detroit, Michigan. Once there, Houdini finally saw a doctor, who urged that he immediately go to the hospital. Houdini refused and, despite a temperature of 102, went on to give his usual performance that night. Only after completing the show did Houdini finally agree to enter the hospital. When doctors operated, they found that his appendix had burst, causing peritonitis, a usually fatal disease in this age before the development of antibiotics. Another operation was later performed, but Houdini was given little hope of surviving. Bess, meanwhile, still suffering from food poisoning, was checked into the same hospital. Believing he was near death, Houdini reportedly shared a secret message with Bess to be used as proof that he was communicating with her from beyond the grave. She would know it was really him if she heard the words “Rosabelle, believe.” “Rosabelle” was the name of a song that Bess had sung at Coney Island in the period when she met Houdini.
Houdini’s brother Theo was at his side when Houdini spoke his last words: “I’m tired of fighting…I guess this thing is going to get me.” Harry Houdini died on the afternoon of Halloween, October 31, 1926.
Houdini’s funeral was held in New York City, where thousands of mourners lined the streets as the funeral procession passed. A representative of the Society of American Magicians broke a wand at the services, beginning a new tradition that has been used for Society members ever since. Houdini was buried at the Machpelah Cemetery in Long Island, New York, beside his parents. Beneath his head was placed a pillow containing his mother’s letters.
Houdini’s collection of over 5,000 books was bequeathed to the Library of Congress. His brother Theo received most of his magic equipment and memorabilia. Theo continued to work as a magician under the name Hardeen; he died in 1945. The bulk of Houdini’s estate went to Bess, who, after paying Houdini’s extensive debts, had enough to live comfortably. For many years Bess tried to contact Houdini through a séance on the anniversary of his death, but died in 1943 without succeeding.
Sunday, March 20, 2011
The Principle of Thermostat
A thermostat is an appliance that regulates the temperature of a system so that the temperature of that system is kept at a particular desired temperature. This desired temperature is described as the 'set point' temperature. The thermostat operates by turning heating devices on or off, to maintain the temperature of a fluid at the set point temperature. The 'main' thermostat refers to the thermostat in a heating system that possesses only one thermostat.
Technological Sensor Methods
- One of the key principles of a main thermostat, and indeed any thermostat, is the technological method by which the thermostat senses the ambient temperature. There are two main techniques by which thermostats can sense their surrounding temperature. These are bimetallic sensors and electronic thermistors. Bimetallic sensors use a strip of two metals joined together that have slightly different expansion rates in response to heat, this results in a bending of the bimetallic strip that can be used to break an electrical circuit at high enough temperatures. Electronic thermistors are an electronic component that increases its electrical resistance with increasing temperature, and as such can be used to break a circuit when a particular temperature is reached.
Feedback
- Thermostats are based on the principle of feedback. The thermistor controls the output of a heating system. The heating system heats a fluid. When the fluid reaches a certain temperature it triggers the thermostat to reduce the heat output, usually by simply switching off the heating system. When the heating system is switched off the temperature of the fluid falls until the thermistor reactivates the heating system. This type of control system is called 'negative feedback' and is a key principle in the design of the main thermostat in many central heating systems.
Digital or Analogue
- Another key principle of main thermostats is whether they are digital or analogue. This key principle of main thermostats is based around the idea that some thermostats work simply by turning a heating system on or off when a particular temperature is reached or constantly adjusting the heat output of the heating system across a range of possible outputs. The former are digital thermostats and the latter are analogue thermostats.
Location
- A key principle of the main thermostat, when that thermostat is controlling a central heating system in your house, is its location in your house. If a main thermostat is poorly located it can lead to high levels of energy inefficiency and poor heating patterns. In a small house a sensible place for the location of a main thermostat is on the staircase or upstairs landing.
World's deadliest disasters ( You will get shocked if you will see )
Tsunami

A small boat gets stuck in a tsunami whirlpool.
Tsunami ploughed into tragic Miyako city, Japan
Waves of tsunami topple trees

Houses swallowed by the tsunami burn in Sendai, Miyagi

Houses swept by a tsunami

Tsunami hits airport in Sendai, Japan
Earthquake

Fractured road, Japan

Twisted railroad, Japan

Collapsed bridge, Guatemala

A rupture forms in road after an earthquake

A massive earthquake struck southwest China

San Andreas Fault, California
Wildfire

San Diego wildfires

Boise forest fire

Wildfires force evacuations in L.A.

Idaho fire
Volcano

Three volcanoes – Mount Semeru, Mount Bromo and Mount Batok in Indonesia

Mount Etna, Italy

Volcanic lightning

Two eruptions

Ash cloud
Tornado

A mother ship cloud formation hovers over Childress, Texas

Violent tornado in northeastern Iowa

Waterspout

Curved tornado

South Dakota tornado

Big tornado
Lightning

City strike

Bolts on the water

CN tower, Canada

Lightning over Miami

Lightning at night, Walton, Nebraska
Other Natural Disasters

Hurricane Ivan

Hurricane winds

Avalanche in Mt. Rainier National Park in Washington

Rock mountain avalanche

A sandstorm engulfs the Saudi capital

Sandstorm strikes Israel

A small boat gets stuck in a tsunami whirlpool.

Tsunami ploughed into tragic Miyako city, Japan

Waves of tsunami topple trees

Houses swallowed by the tsunami burn in Sendai, Miyagi

Houses swept by a tsunami

Tsunami hits airport in Sendai, Japan
Earthquake

Fractured road, Japan

Twisted railroad, Japan

Collapsed bridge, Guatemala

A rupture forms in road after an earthquake

A massive earthquake struck southwest China

San Andreas Fault, California
Wildfire

San Diego wildfires

Boise forest fire

Wildfires force evacuations in L.A.

Idaho fire
Volcano

Three volcanoes – Mount Semeru, Mount Bromo and Mount Batok in Indonesia

Mount Etna, Italy

Volcanic lightning

Two eruptions

Ash cloud
Tornado

A mother ship cloud formation hovers over Childress, Texas

Violent tornado in northeastern Iowa

Waterspout

Curved tornado

South Dakota tornado

Big tornado
Lightning

City strike

Bolts on the water

CN tower, Canada

Lightning over Miami

Lightning at night, Walton, Nebraska
Other Natural Disasters

Hurricane Ivan

Hurricane winds

Avalanche in Mt. Rainier National Park in Washington

Rock mountain avalanche

A sandstorm engulfs the Saudi capital

Sandstorm strikes Israel
How to create a stored procedure(SQL Server Management Studio)
This topic describes how to create a Transact-SQL stored procedure by using Object Explorer in SQL Server Management Studio and provides an example that creates a simple stored procedure in the AdventureWorks2008R2 database.
To create a stored procedure
- In Object Explorer, connect to an instance of Database Engine and then expand that instance.
- Expand Databases, expand the database in which the stored procedure belongs, and then expand Programmability.
- Right-click Stored Procedures, and then click New Stored Procedure.
- On the Query menu, click Specify Values for Template Parameters.
- In the Specify Values for Template Parameters dialog box, the Value column contains suggested values for the parameters. Accept the values or replace them with new values, and then click OK.
- In the query editor, replace the SELECT statement with the statements for your procedure.
- To test the syntax, on the Query menu, click Parse.
- To create the stored procedure, on the Query menu, click Execute.
- To save the script, on the File menu, click Save. Accept the file name or replace it with a new name, and then click Save.
To create a stored procedure example
- In Object Explorer, connect to an instance of Database Engine and then expand that instance.
- Expand Databases, expand the AdventureWorks2008R2 database, and then expand Programmability.
- Right-click Stored Procedures, and then click New Stored Procedure.
- On the Query menu, click Specify Values for Template Parameters.
- In the Specify Values for Template Parameters dialog box, enter the following values for the parameters shown.
Parameter Value Author Your name Create Date Today's date Description Returns employee data. Procedure_name HumanResources.uspGetEmployees @Param1 @LastName @Datatype_For_Param1 nvarchar(50) Default_Value_For_Param1 NULL @Param2 @FirstName @Datatype_For_Param2 nvarchar(50) Default_Value_For_Param2 NULL - Click OK.
- In the query editor, replace the SELECT statement with the following statement:
SELECT FirstName, LastName, JobTitle, Department FROM HumanResources.vEmployeeDepartment WHERE FirstName = @FirstName AND LastName = @LastName;- To test the syntax, on the Query menu, click Parse. If an error message is returned, compare the statements with the information above and correct as needed.
- To create the stored procedure, on the Query menu, click Execute.
- To save the script, on the File menu, click Save. Enter a new file name, and then click Save.
- To run the stored procedure, on the toolbar, click New Query.
- In the query window, enter the following statements:
USE AdventureWorks2008R2; GO EXECUTE HumanResources.uspGetEmployees @FirstName = N'Diane', @LastName = N'Margheim';
On the Query menu, click Execute. GO
Friday, March 18, 2011
IP Internet protocol
Internet Protocol: IP Addresses
Every machine on the Internet has a unique identifying number, called an IP Address. The IP stands for Internet Protocol, which is the language that computers use to communicate over the Internet. A protocol is the pre-defined way that someone who wants to use a service talks with that service. The "someone" could be a person, but more often it is a computer program like a Web browser.A typical IP address looks like this:
Out of the almost 4.3 billion possible combinations, certain values are restricted from use as typical IP addresses. For example, the IP address 0.0.0.0 is reserved for the default network and the address 255.255.255.255 is used for broadcasts.
The octets serve a purpose other than simply separating the numbers. They are used to create classes of IP addresses that can be assigned to a particular business, government or other entity based on size and need. The octets are split into two sections: Net and Host. The Net section always contains the first octet. It is used to identify the network that a computer belongs to. Host (sometimes referred to as Node) identifies the actual computer on the network. The Host section always contains the last octet. There are five IP classes plus certain special addresses.
Internet Protocol: Domain Name System
When the Internet was in its infancy, it consisted of a small number of computers hooked together with modems and telephone lines. You could only make connections by providing the IP address of the computer you wanted to establish a link with. For example, a typical IP address might be 216.27.22.162. This was fine when there were only a few hosts out there, but it became unwieldy as more and more systems came online.The first solution to the problem was a simple text file maintained by the Network Information Center that mapped names to IP addresses. Soon this text file became so large it was too cumbersome to manage. In 1983, the University of Wisconsin created the Domain Name System (DNS), which maps text names to IP addresses automatically
More information will be given in few days so please stay tune
Chemical Equilibrium
Definition of Chemical Equilibrium
Even though the reactants are constantly forming products and vice-versa the amount of reactants and products does become steady. When the net change of the products and reactants is zero the reaction has reached equilibrium. The equilibrium is a dynamic equilibrium. The definition for a dynamic equilibrium is when the amount of products and reactants are constant. (They are not equal but constant. Also, both reactions are still occurring.)
Equilibrium Constant

For example, determining the equilibrium constant of the following equation can be accomplished by using the Kc equation.
Using the following equation, calculate the equilibrium constant.
N2(g) + 3H2(g) <--> 2NH3(g)
A one-liter vessel contains 1.60 moles NH3, .800 moles N2, and 1.20 moles of H2. What is the equilibrium constant?

Answer: 1.85
Le Chatelier's Principle
- Changing concentrations by adding or removing products or reactants to the reaction vessel.
- Changing partial pressure of gaseous reactants and products.
- Changing the temperature.
Example involving change of concentration:
In the equationIf you add more O2(g) the equilibrium shifts to the right producing more NO2(g)
If you add more NO2(g) the equilibrium shifts to the left producing more NO(g) and O2(g)
Example involving pressure change:
In the equationExample involving temperature change:
In the equationIUPAC Nomenclature for organic chemistry
Nomenclature
Naming Organic Compounds
As organic chemistry grew and developed, many compounds were given trivial names, which are now commonly used and recognized. Some examples are:
| Name | Methane | Butane | Acetone | Toluene | Acetylene | Ethyl Alcohol |
|---|---|---|---|---|---|---|
| Formula | CH4 | C4H10 | CH3COCH3 | CH3C6H5 | C2H2 | C2H5OH |
The IUPAC Systematic Approach to Nomenclature
The IUPAC nomenclature system is a set of logical rules devised and used by organic chemists to circumvent problems caused by arbitrary nomenclature. Knowing these rules and given a structural formula, one should be able to write a unique name for every distinct compound. Likewise, given a IUPAC name, one should be able to write a structural formula. In general, an IUPAC name will have three essential features:
• A suffix or other element(s) designating functional groups that may be present in the compound.
• Names of substituent groups, other than hydrogen, that complete the molecular structure.
An excellent presentation of organic nomenclature is provided on a Nomenclature Page. created by Dave Woodcock.
Alkanes |
|---|
Alkanes
Hydrocarbons having no double or triple bond functional groups are classified as alkanes or cycloalkanes, depending on whether the carbon atoms of the molecule are arranged only in chains or also in rings. Although these hydrocarbons have no functional groups, they constitute the framework on which functional groups are located in other classes of compounds, and provide an ideal starting point for studying and naming organic compounds. The alkanes and cycloalkanes are also members of a larger class of compounds referred to as aliphatic. Simply put, aliphatic compounds are compounds that do not incorporate any aromatic rings in their molecular structure.The following table lists the IUPAC names assigned to simple continuous-chain alkanes from C-1 to C-10. A common "ane" suffix identifies these compounds as alkanes. Longer chain alkanes are well known, and their names may be found in many reference and text books. The names methane through decane should be memorized, since they constitute the root of many IUPAC names. Fortunately, common numerical prefixes are used in naming chains of five or more carbon atoms.
| Name | Molecular Formula | Structural Formula | Isomers | Name | Molecular Formula | Structural Formula | Isomers | |
|---|---|---|---|---|---|---|---|---|
| methane | CH4 | CH4 | 1 | hexane | C6H14 | CH3(CH2)4CH3 | 5 | |
| ethane | C2H6 | CH3CH3 | 1 | heptane | C7H16 | CH3(CH2)5CH3 | 9 | |
| propane | C3H8 | CH3CH2CH3 | 1 | octane | C8H18 | CH3(CH2)6CH3 | 18 | |
| butane | C4H10 | CH3CH2CH2CH3 | 2 | nonane | C9H20 | CH3(CH2)7CH3 | 35 | |
| pentane | C5H12 | CH3(CH2)3CH3 | 3 | decane | C10H22 | CH3(CH2)8CH3 | 75 |
(ii) A uniform variation of this kind in a series of compounds is called homologous.
(iii) These formulas all fit the CnH2n+2 rule. This is also the highest possible H/C ratio for a stable hydrocarbon.
(iv) Since the H/C ratio in these compounds is at a maximum, we call them saturated (with hydrogen).
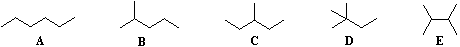
The IUPAC system requires first that we have names for simple unbranched chains, as noted above, and second that we have names for simple alkyl groups that may be attached to the chains. Examples of some common alkyl groups are given in the following table. Note that the "ane" suffix is replaced by "yl" in naming groups. The symbol R is used to designate a generic (unspecified) alkyl group.
| Group | CH3– | C2H5– | CH3CH2CH2– | (CH3)2CH– | CH3CH2CH2CH2– | (CH3)2CHCH2– | CH3CH2CH(CH3)– | (CH3)3C– | R– |
| Name | Methyl | Ethyl | Propyl | Isopropyl | Butyl | Isobutyl | sec-Butyl | tert-Butyl | Alkyl |
IUPAC Rules for Alkane Nomenclature2. Identify and name groups attached to this chain. 3. Number the chain consecutively, starting at the end nearest a substituent group. 4. Designate the location of each substituent group by an appropriate number and name. 5. Assemble the name, listing groups in alphabetical order. The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing. |
Cycloalkanes |
|---|
Cycloalkanes
Cycloalkanes have one or more rings of carbon atoms. The simplest examples of this class consist of a single, unsubstituted carbon ring, and these form a homologous series similar to the unbranched alkanes. The IUPAC names of the first five members of this series are given in the following table. The last (yellow shaded) column gives the general formula for a cycloalkane of any size. If a simple unbranched alkane is converted to a cycloalkane two hydrogen atoms, one from each end of the chain, must be lost. Hence the general formula for a cycloalkane composed of n carbons is CnH2n. Although a cycloalkane has two fewer hydrogens than the equivalent alkane, each carbon is bonded to four other atoms so such compounds are still considered to be saturated with hydrogen.| Name | Cyclopropane | Cyclobutane | Cyclopentane | Cyclohexane | Cycloheptane | Cycloalkane |
|---|---|---|---|---|---|---|
| Molecular Formula | C3H6 | C4H8 | C5H10 | C6H12 | C7H14 | CnH2n |
| Structural Formula | 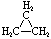 | 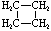 | 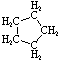 | 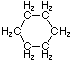 | 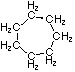 | (CH2)n |
| Line Formula |  |  | 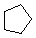 | 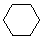 | 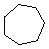 | 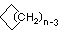 |
IUPAC Rules for Cycloalkane Nomenclature2. If the alkyl substituent is large and/or complex, the ring may be named as a substituent group on an alkane. 3. If two different substituents are present on the ring, they are listed in alphabetical order, and the first cited substituent is assigned to carbon #1. The numbering of ring carbons then continues in a direction (clockwise or counter-clockwise) that affords the second substituent the lower possible location number. 4. If several substituents are present on the ring, they are listed in alphabetical order. Location numbers are assigned to the substituents so that one of them is at carbon #1 and the other locations have the lowest possible numbers, counting in either a clockwise or counter-clockwise direction. 5. The name is assembled, listing groups in alphabetical order and giving each group (if there are two or more) a location number. The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing. |
Small rings, such as three and four membered rings, have significant angle strain resulting from the distortion of the sp3 carbon bond angles from the ideal 109.5º to 60º and 90º respectively. This angle strain often enhances the chemical reactivity of such compounds, leading to ring cleavage products. It is also important to recognize that, with the exception of cyclopropane, cycloalkyl rings are not planar (flat). The three dimensional shapes assumed by the common rings (especially cyclohexane and larger rings) are described and discussed in the Conformational Analysis Section.
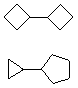
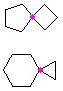
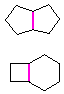
Hydrocarbons having more than one ring are common, and are referred to as bicyclic (two rings), tricyclic (three rings) and in general, polycyclic compounds. The molecular formulas of such compounds have H/C ratios that decrease with the number of rings. In general, for a hydrocarbon composed of n carbon atoms associated with m rings the formula is: CnH(2n + 2 - 2m). The structural relationship of rings in a polycyclic compound can vary. They may be separate and independent, or they may share one or two common atoms. Some examples of these possible arrangements are shown in the following table.
Examples of Isomeric C8H14 Bicycloalkanes
| Isolated Rings | Spiro Rings | Fused Rings | Bridged Rings |
|---|---|---|---|
| No common atoms | One common atom | One common bond | Two common atoms |
 |  |  | 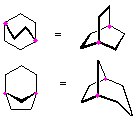 |
Subscribe to:
Posts (Atom)



